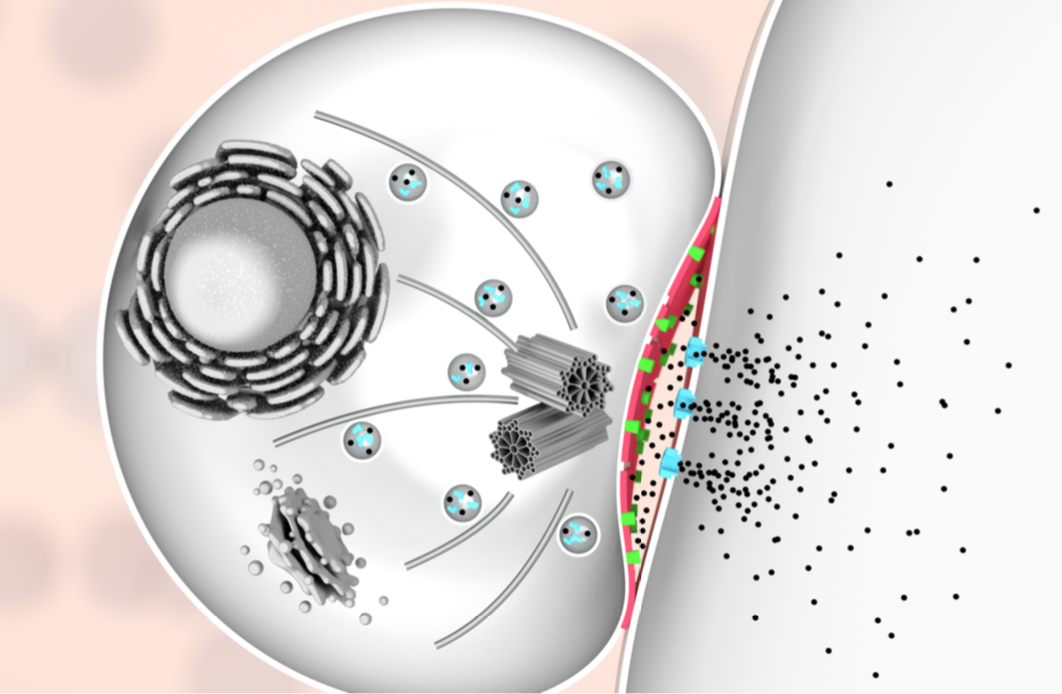
 To clear our body from cancerous and virus-infected cells, our immune system relies on a type of white blood cells called killer lymphocytes. Once they have identified their targets, these white blood cells release a toxic potion of proteins. In a study published in Nature Communications, we have found what prevents the white blood cells from being killed by their own actions. Our findings could help to explain why some tumours are more resistant than others to recently developed cancer immunotherapies. Cytotoxic lymphocytes rid the body of disease by punching holes in rogue cells and by injecting poisonous enzymes inside. Remarkably, they can do this many times in a row, without harming themselves. Our study, carried out in tight collaboration with Ilia Voskoboinik’s lab at the Peter MacCallum Cancer Centre in Melbourne, discovers the protective role of molecular order and electric charge in the cell envelope of the white blood cells. We made this discovery by studying perforin, which is the protein responsible for the hole-punching. We found that perforin’s attachment to the cell surface strongly depends on the order and packing of the molecules – so-called lipids – in the membrane that surrounds and protects the white blood cells. More order and tighter packing of the lipid molecules led to less perforin binding, and when we artificially disrupted the order of this lipid in the white blood cells, the cells became more sensitive to perforin. However, we also found that when the white blood cells were exposed to so much perforin that some of it stuck to their surface, the bound perforin still failed to kill the white blood cells, indicating that there must be another layer of protection. This turned out to be the negative charge of some lipid molecules sent to the cell surface, which bound the remaining perforin and blocked it from damaging the cell. According to Adrian Hodel, joint first author and PhD student in our lab: “We have long known that local lipid order can change how cells communicate which each other, but it was rather surprising that the precise physical membrane properties can also provide such an important layer of protection against molecular hole-punchers.” In Melbourne, joint first author Jesse Rudd-Schmidt, who focused on the characterisation of the white blood cells in Ilia Voskoboinik’s laboratory, said: “What we have found helps to explain how our immune system can be so effective in killing rogue cells. We are now also keen to investigate if cancer cells may use similar protection to avoid being killed by immune cells, which would then explain some of the large variability in patient response to cancer immunotherapies.” The work was kindly funded by the Australian National Health and Medical Research Council and by the UK Biotechnology and Biological Sciences Research Council and Engineering and Physical Sciences Research Council, and by the Sackler Foundation. Comments are closed.
|

 RSS Feed
RSS Feed